Research Article
Balam singh Bisht1*, Darshan Singh1, Chandra S. Mathela1 and Amit Panwar2
Abstract
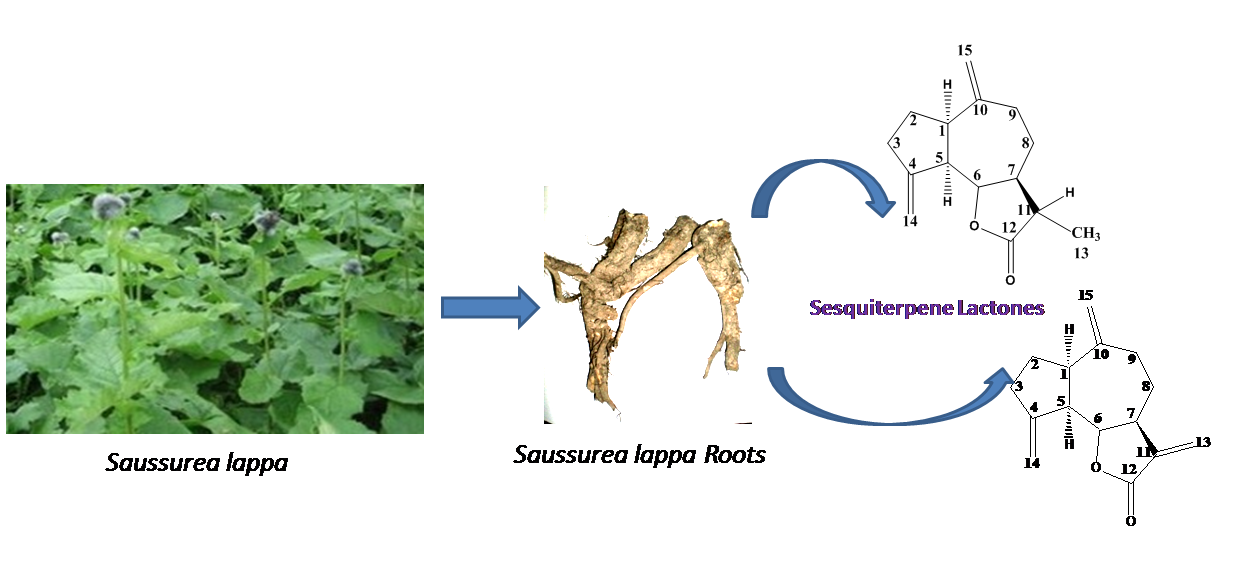
Dihydrodehydrocostuslactone
and dehydrocostuslactone were isolated from the roots of Saussurea lappa syn. Saussurea
costus as two major sesquiterpene lactones. The structures of the
isolates were determined using their MS and NMR (1H,
13C) data. GC-MS of root oil and extracts (diethyl ether and
hexane) showed about three dozen constituents, of which 33 compounds were
identified. The oil/ extract was dominated by the presence of
dehydrocostuslactone and/ or dihydrodehydro-costuslactone besides
10-epi-γ-eudesmol and 1,8-cineol as other constituents. The root oil, ether and hexane extracts
were tested for antimicrobial activity against five bacterial species and two
fungi. The extracts and the root oil showed moderate activity in controlling A.
hydrophila, B. subtilis, S. candidus and E. coli.
Abstract Keywords
Saussurea
lappa,
Asteraceae, Sesquiterpene lactones, Dihydrodehydrocostuslactone,
Dehydrocostuslactone, Antimicrobial activity.
Abstract
Dihydrodehydrocostuslactone
and dehydrocostuslactone were isolated from the roots of Saussurea lappa syn. Saussurea
costus as two major sesquiterpene lactones. The structures of the
isolates were determined using their MS and NMR (1H,
13C) data. GC-MS of root oil and extracts (diethyl ether and
hexane) showed about three dozen constituents, of which 33 compounds were
identified. The oil/ extract was dominated by the presence of
dehydrocostuslactone and/ or dihydrodehydro-costuslactone besides
10-epi-γ-eudesmol and 1,8-cineol as other constituents. The root oil, ether and hexane extracts
were tested for antimicrobial activity against five bacterial species and two
fungi. The extracts and the root oil showed moderate activity in controlling A.
hydrophila, B. subtilis, S. candidus and E. coli.
Keywords
Saussurea
lappa,
Asteraceae, Sesquiterpene lactones, Dihydrodehydrocostuslactone,
Dehydrocostuslactone, Antimicrobial activity.
References
1.
Polunin, O.;
Stainton, A. Flowers of the Himalaya,
Oxford University Press, New Delhi, India, 1984.
2.
Pandey, M.M.;
Rastogi, S.; Rawat, A.K.S. Saussurea
costus: Botanical, chemical and pharmacological review of an ayurvedic
medicinal plant. J. Ethnopharmacol.
2007, 110, 379-390.
3.
Julianti, T.; Hata, Y.; Zimmermann,
S.; Kaiser, M.; Hamburger, M.; Adams, M. Antitrypanosomal sesquiterpene
lactones from Saussurea costus. Fitoterapia.
2011, 82, 955-959.
4.
Chhabra, B.R.;
Gupta, S.; Jain, M., Kalsi, P.S. Sesquiterpene lactones from Saussurea lappa.
Phytochem. 1998, 49, 801-804.
5.
Kraker, J.W.;
Franssen, M.C.R.; Groot, A.; Shibata, T.; Harro, J.B. Germacrenes from fresh
costus roots. Phytochem. 2001, 58,
481-487.
6.
Yu, H.H.; Lee,
J.S.; Lee, K.H.; Kim, K.Y.; You, Y.O. Saussurea
lappa inhibits the growth, acid production, adhesion, and water-insoluble
glucan synthesis of Streptococcus mutans. J. Ethnopharmacol. 2007,111,
413-417.
7.
Li, Y.;
Xu, C.; Zang, Q.; Liu, J.Y.; Tan, R.X. In vitro anti-Helicobacter pylori action
of 30 Chinese herbal medicines used to treat ulcer diseases. J. Ethnopharmacol. 2005, 98, 329-333.
8.
Chopra, R.N.;
Nayer, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants, 3rd ed.; Council
of Scientific and Industrial Research, New Delhi, 1992, 246.
9.
Chopra, R.N.;
Nayer, S.L.; Chopra, I.C. Glossary of Indian Medicinal Plants, New Delhi, 1956,
222.
10. Kirtikar, K.; Basu, B.D.; Basu,
L.M. Indian Medicinal Plants, Allahabad, 1993,
420.
11. Sarin, Y.K. Illustrated Manual of Herbal Drugs used in
Ayurveda, New Delhi, 1996, 62.
12. Kalsi, P.S.; Kumar, S.; Jawanda, G.S.; Chhabra, B.R.
Guainolides from Saussurea lappa, Phytochem. 1995, 40,
1713-1715.
13.
Dhillon,
R.S.; Kalsi, P.S.; Singh, W.P.; Gautam, V.K.; Chhabra, B.R. Guaianolide from Saussurea lappa. Phytochem.
1987, 26, 1209-1210.
14. Chang, K.M.; Kim, G.H. Comparison of
volatile aroma components from Saussurea lappa C.B. Clarke root oils. J. Food Sci. Nutr. 2008, 13, 128-133.
15.
Robinson, A.; Vijay, K. T.;
Sreedhar, E.; Naidu, V.G.M.; Krishna, S.R.; Babu, K.S.; Srinivas, P.V.; Rao, J.M.
A new sesquiterpene lactone from the roots of Saussurea lappa: structure–anticancer
activity study. Bio. Med. Chem. Lett. 2008, 18,
4015-4017.
16. Chhabra, B.R.; Gupta, S.; Jain, M., Kalsi, P.S. Sesquiterpene
lactones from Saussurea lappa. Phytochem. 1998, 49, 801-804.
17. Marta, N.; Morais, R.; Gafner, S.;
Stoeckli, E. H.; Hotettmann, K. New sesquiterpene lactones from Portuguese
liverwort Targionia lobeeriana. Phytochem. 1999, 50, 967-972.
18. Shoji, N.; Umeyama, A.; Saito, N.;
Takemoto, T. Vasoactive substances from Saussurea lappa. J. Nat. Prod. 1986, 49(6), 1112-1113.
19. Wu, T. S.; Leu, Y. L.; Kuoh, C. S.;
Jiang S. D.; Chen C. F.; Lee K. H. Cytotoxic principles from Saussurea lappa and Corydalis Turtshaninovii f. Yanhusuo. J. Chi. Chem. Soc. 1997, 44, 357-359.
20. Raghav, C.S.; Suneja, P.; Mohan, J.; Srivastava, V.K.;
Singh, M.; Naik, S.N. Investigation of costus root oil composition. Ind. J. Pla. Gen. Res. 2011, 4, 88-91.
21. Rios, J. L.; Recio, M.C.; Vilar, A. Screening methods for natural products
with antimicrobial activity: a review of the literature. J. Ethnopharmacol. 1988, 23, 127-149.
22. Mathela, C.S.; Chanotiya, C.S.; Sammal, S.S.; Pant,
A.K.; Pandey, S. Compositional diversity of terpenoids in the Himalayan Valeriana genera. Chem. Biod. 2005, 2(9), 1174-1182.
23. Adams, R.P. Identification of essential oil
components by Gas Chromatography/ Mass Spectrometry; 4th ed.;
Allured Publishing: Carol Stream, IL, USA, 2007.
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Abstract
Dihydrodehydrocostuslactone
and dehydrocostuslactone were isolated from the roots of Saussurea lappa syn. Saussurea
costus as two major sesquiterpene lactones. The structures of the
isolates were determined using their MS and NMR (1H,
13C) data. GC-MS of root oil and extracts (diethyl ether and
hexane) showed about three dozen constituents, of which 33 compounds were
identified. The oil/ extract was dominated by the presence of
dehydrocostuslactone and/ or dihydrodehydro-costuslactone besides
10-epi-γ-eudesmol and 1,8-cineol as other constituents. The root oil, ether and hexane extracts
were tested for antimicrobial activity against five bacterial species and two
fungi. The extracts and the root oil showed moderate activity in controlling A.
hydrophila, B. subtilis, S. candidus and E. coli.
Abstract Keywords
Saussurea
lappa,
Asteraceae, Sesquiterpene lactones, Dihydrodehydrocostuslactone,
Dehydrocostuslactone, Antimicrobial activity.
This work is licensed under the
Creative Commons Attribution
4.0
License (CC BY-NC 4.0).
Editor-in-Chief
This work is licensed under the
Creative Commons Attribution 4.0
License.(CC BY-NC 4.0).


